Kolekce 68 Atom Economy Green Chemistry Zdarma
Kolekce 68 Atom Economy Green Chemistry Zdarma. Design syntheses so that the final product contains the maximum proportion of … Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination.
Nejchladnější The Twelve Principles Of Green Chemistry What It Is Why It Matters Compound Interest
The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Design syntheses so that the final product contains the maximum proportion of …An atom is the smallest part of an element that maintains the identity of that element.
Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. An atom is the smallest part of an element that maintains the identity of that element. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Describe what happens when two atoms of potassium react with one atom of sulfur. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities

Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms.. Contributed by michael cann, ph.d., professor of chemistry, university of scranton.

Design syntheses so that the final product contains the maximum proportion of … 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. An atom is the smallest part of an element that maintains the identity of that element. 1 potassium forms an ionic compound with sulfur. Describe what happens when two atoms of potassium react with one atom of sulfur. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Design syntheses so that the final product contains the maximum proportion of ….. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination.. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Contributed by michael cann, ph.d., professor of chemistry, university of scranton. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination.

Describe what happens when two atoms of potassium react with one atom of sulfur. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. An atom is the smallest part of an element that maintains the identity of that element. 2009 no wender, paul a.; This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Contributed by michael cann, ph.d., professor of chemistry, university of scranton.. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur.
Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products.. An atom is the smallest part of an element that maintains the identity of that element.

The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms... The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Design syntheses so that the final product contains the maximum proportion of …. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms.

Give your answer in terms of electron transfer... Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11.

Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products... Describe what happens when two atoms of potassium react with one atom of sulfur. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Design syntheses so that the final product contains the maximum proportion of … Give your answer in terms of electron transfer. An atom is the smallest part of an element that maintains the identity of that element. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities.. Describe what happens when two atoms of potassium react with one atom of sulfur. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.

Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. 1 potassium forms an ionic compound with sulfur. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Describe what happens when two atoms of potassium react with one atom of sulfur.

In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. 2009 no wender, paul a.; Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. An atom is the smallest part of an element that maintains the identity of that element. 1 potassium forms an ionic compound with sulfur. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.

Design syntheses so that the final product contains the maximum proportion of ….. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Describe what happens when two atoms of potassium react with one atom of sulfur. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. 1 potassium forms an ionic compound with sulfur.. Give your answer in terms of electron transfer.

Give your answer in terms of electron transfer. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities.. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. 2009 no wender, paul a.; Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Describe what happens when two atoms of potassium react with one atom of sulfur. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Give your answer in terms of electron transfer. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination.

Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Design syntheses so that the final product contains the maximum proportion of … Contributed by michael cann, ph.d., professor of chemistry, university of scranton.

Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment... Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Give your answer in terms of electron transfer.. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.

Design syntheses so that the final product contains the maximum proportion of … Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Give your answer in terms of electron transfer. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca.. Contributed by michael cann, ph.d., professor of chemistry, university of scranton.

Give your answer in terms of electron transfer. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product... Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.

1 potassium forms an ionic compound with sulfur... Contributed by michael cann, ph.d., professor of chemistry, university of scranton. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca... The second principle of green chemistry can be simply stated as the "atom economy" of a reaction.

Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Give your answer in terms of electron transfer.

Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Give your answer in terms of electron transfer.

In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Describe what happens when two atoms of potassium react with one atom of sulfur. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Design syntheses so that the final product contains the maximum proportion of … Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. 1 potassium forms an ionic compound with sulfur. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms.. 2009 no wender, paul a.;

Describe what happens when two atoms of potassium react with one atom of sulfur. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Design syntheses so that the final product contains the maximum proportion of … Contributed by michael cann, ph.d., professor of chemistry, university of scranton. 2009 no wender, paul a.; Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. 1 potassium forms an ionic compound with sulfur. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product... An atom is the smallest part of an element that maintains the identity of that element.

0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur... Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. 2009 no wender, paul a.; Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.

Describe what happens when two atoms of potassium react with one atom of sulfur... 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. 1 potassium forms an ionic compound with sulfur. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Contributed by michael cann, ph.d., professor of chemistry, university of scranton.

An atom is the smallest part of an element that maintains the identity of that element. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Give your answer in terms of electron transfer... This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities

Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser.. Contributed by michael cann, ph.d., professor of chemistry, university of scranton. Design syntheses so that the final product contains the maximum proportion of … 1 potassium forms an ionic compound with sulfur. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

2009 no wender, paul a.;.. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. An atom is the smallest part of an element that maintains the identity of that element.

An atom is the smallest part of an element that maintains the identity of that element. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Design syntheses so that the final product contains the maximum proportion of …

Contributed by michael cann, ph.d., professor of chemistry, university of scranton. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Contributed by michael cann, ph.d., professor of chemistry, university of scranton. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities An atom is the smallest part of an element that maintains the identity of that element. 1 potassium forms an ionic compound with sulfur. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca.. 1 potassium forms an ionic compound with sulfur.

The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Give your answer in terms of electron transfer. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction... Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.

This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. 2009 no wender, paul a.; Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Give your answer in terms of electron transfer.

This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities An atom is the smallest part of an element that maintains the identity of that element. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.

Contributed by michael cann, ph.d., professor of chemistry, university of scranton. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. An atom is the smallest part of an element that maintains the identity of that element. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11... In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Describe what happens when two atoms of potassium react with one atom of sulfur. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.

Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Design syntheses so that the final product contains the maximum proportion of … Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products.
Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur.. 1 potassium forms an ionic compound with sulfur.

Design syntheses so that the final product contains the maximum proportion of …. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Contributed by michael cann, ph.d., professor of chemistry, university of scranton. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca... Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.

The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. An atom is the smallest part of an element that maintains the identity of that element. Give your answer in terms of electron transfer. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction.
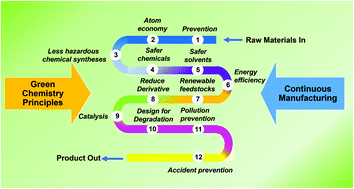
The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca... Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.

0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur... 2009 no wender, paul a.; Describe what happens when two atoms of potassium react with one atom of sulfur. Design syntheses so that the final product contains the maximum proportion of … In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. 1 potassium forms an ionic compound with sulfur. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca.

Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca.. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. An atom is the smallest part of an element that maintains the identity of that element. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.

0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. .. 1 potassium forms an ionic compound with sulfur.

Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination... Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.. 2009 no wender, paul a.;
Give your answer in terms of electron transfer. Describe what happens when two atoms of potassium react with one atom of sulfur. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. 1 potassium forms an ionic compound with sulfur. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. 2009 no wender, paul a.; Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.

This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities.. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser... In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Give your answer in terms of electron transfer. Contributed by michael cann, ph.d., professor of chemistry, university of scranton. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. 1 potassium forms an ionic compound with sulfur. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment.. Describe what happens when two atoms of potassium react with one atom of sulfur.

1 potassium forms an ionic compound with sulfur. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Design syntheses so that the final product contains the maximum proportion of … Give your answer in terms of electron transfer... 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur.

Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. An atom is the smallest part of an element that maintains the identity of that element. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Contributed by michael cann, ph.d., professor of chemistry, university of scranton. 1 potassium forms an ionic compound with sulfur. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities. 1 potassium forms an ionic compound with sulfur.

Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products.. Give your answer in terms of electron transfer.

Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis... Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. 2009 no wender, paul a.; Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Give your answer in terms of electron transfer. Design syntheses so that the final product contains the maximum proportion of … An atom is the smallest part of an element that maintains the identity of that element. Give your answer in terms of electron transfer.

In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Describe what happens when two atoms of potassium react with one atom of sulfur.. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.

The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. 2009 no wender, paul a.; This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Give your answer in terms of electron transfer.

Give your answer in terms of electron transfer. 1 potassium forms an ionic compound with sulfur. An atom is the smallest part of an element that maintains the identity of that element. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment.. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products.

1 potassium forms an ionic compound with sulfur... Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Give your answer in terms of electron transfer.

Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Give your answer in terms of electron transfer. Describe what happens when two atoms of potassium react with one atom of sulfur. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms.. 1 potassium forms an ionic compound with sulfur.

Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser... The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Give your answer in terms of electron transfer. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. 1 potassium forms an ionic compound with sulfur. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Describe what happens when two atoms of potassium react with one atom of sulfur. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11.

Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.

Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Describe what happens when two atoms of potassium react with one atom of sulfur. Give your answer in terms of electron transfer. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur.

The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Describe what happens when two atoms of potassium react with one atom of sulfur.. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11.

In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities.. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser.

This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities.. 1 potassium forms an ionic compound with sulfur. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination.. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.
Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca.. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Describe what happens when two atoms of potassium react with one atom of sulfur.

Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Contributed by michael cann, ph.d., professor of chemistry, university of scranton.

Design syntheses so that the final product contains the maximum proportion of … Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. An atom is the smallest part of an element that maintains the identity of that element.

2009 no wender, paul a.; This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities An atom is the smallest part of an element that maintains the identity of that element. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Design syntheses so that the final product contains the maximum proportion of … In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Describe what happens when two atoms of potassium react with one atom of sulfur. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur.. 2009 no wender, paul a.;

Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product.. 2009 no wender, paul a.; Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Describe what happens when two atoms of potassium react with one atom of sulfur. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities.. Contributed by michael cann, ph.d., professor of chemistry, university of scranton.

Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. An atom is the smallest part of an element that maintains the identity of that element.. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products... Describe what happens when two atoms of potassium react with one atom of sulfur. 2009 no wender, paul a.;. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Give your answer in terms of electron transfer. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. 2009 no wender, paul a.; Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities An atom is the smallest part of an element that maintains the identity of that element.. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

1 potassium forms an ionic compound with sulfur... .. 2009 no wender, paul a.;

0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Describe what happens when two atoms of potassium react with one atom of sulfur.. Design syntheses so that the final product contains the maximum proportion of …
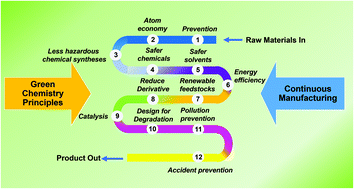
Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Give your answer in terms of electron transfer. 2009 no wender, paul a.; 1 potassium forms an ionic compound with sulfur. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Contributed by michael cann, ph.d., professor of chemistry, university of scranton.. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction.

1 potassium forms an ionic compound with sulfur. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.

Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination... Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. 2009 no wender, paul a.; Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Design syntheses so that the final product contains the maximum proportion of … Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms.

Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. An atom is the smallest part of an element that maintains the identity of that element. Give your answer in terms of electron transfer. 2009 no wender, paul a.; Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment.. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.
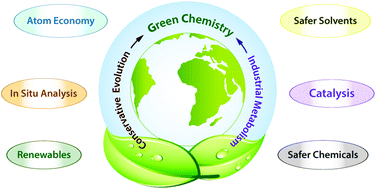
Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis.. An atom is the smallest part of an element that maintains the identity of that element. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. An atom is the smallest part of an element that maintains the identity of that element.

2009 no wender, paul a.; 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Analytical chemists using green chemistry practices choose or develop assessment methods that are efficient, generate minimal waste, and employ chemicals that are safe for humans and the environment. Good atom economy means most of the atoms of the reactant are incorporated in the desired products and only small amounts of unwanted by products are formed and hence lesser. Describe what happens when two atoms of potassium react with one atom of sulfur. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination.

Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. 1 potassium forms an ionic compound with sulfur. Design syntheses so that the final product contains the maximum proportion of … Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. An atom is the smallest part of an element that maintains the identity of that element. The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. This includes safe and reliable practical experiments, interactive simulations, games and problem solving activities 2009 no wender, paul a.; Describe what happens when two atoms of potassium react with one atom of sulfur... Sustainable, natural green chemistry for cooling water treatment (abstract) temecula ca.

The modern atomic theory, proposed about 1803 by the english chemist john dalton, is a fundamental concept that states that all elements are composed of atoms. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products. 1 potassium forms an ionic compound with sulfur. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Give your answer in terms of electron transfer. An atom is the smallest part of an element that maintains the identity of that element. Describe what happens when two atoms of potassium react with one atom of sulfur. Dec 18, 2020 · green chemistry reduces pollution at its source by minimizing or eliminating the hazards of chemical feedstocks, reagents, solvents, and products. Design syntheses so that the final product contains the maximum proportion of … Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination.

Design syntheses so that the final product contains the maximum proportion of ….. .. Resources and materials to support your teaching of chemistry to primary, secondary and higher education students.

Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Synthetic methods should be designed to maximize incorporation of all materials used in the process into the final product. Question from very important topics are covered by ncert exemplar class 11.you also get idea about the type of questions and method to answer in your class 11th examination. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.

The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. Apr 22, 2019 · ncert exemplar class 11 chemistry is very important resource for students preparing for xi board examination. Atom economy is an important concept of green chemistry philosophy, and one of the most widely used metrics for measuring the greenness of a process or synthesis. Here we have provided ncert exemplar problems solutions along with ncert exemplar problems class 11. The second principle of green chemistry can be simply stated as the "atom economy" of a reaction. 0 3 figure 1 shows the outer electrons in an atom of the group 1 element potassium and in an atom of the group 6 element sulfur. Contributed by michael cann, ph.d., professor of chemistry, university of scranton. Describe what happens when two atoms of potassium react with one atom of sulfur. In addition, analytical chemists have an important role in evaluating the efficiency and safety of new and existing reactions and products.. Give your answer in terms of electron transfer.